Many students get goose bumps when asked to study Chemistry – often dreading Organic Chemistry – hundreds of formulae, periodic table, etc. In short, the carbon and its combinations appear tough to even remember, let aside clubbing them with other compounds. But just like Maths requires clarity of fundamentals and everything gets simplified, so does Organic Chemistry. Once you get your fundamentals right, believe us, you will never forget it all your life. It is that simple.
So let’s get our fundamentals clear, from the beginning:
What’s Chemistry?
Chemistry is a branch of Science which deals with behaviour of substances in nature. Substances show physical and chemical behaviour; and they all become a part of Chemistry.
All substances on earth exist in the elementary form or compound or complex form. Elementary forms are the simplest forms of substances with no other constituent. These are called elements and there are more than a hundred elements known so far. The whole matter around is formed of only these elements, in various combined forms.
Every element is made up of small identical particles called atoms which are the combining agents of elements. These elements are grouped and arranged systematically in Periodic table and the students in secondary class and onwards are required to know in detail about the arrangement of elements, which covers a great part of details of behaviour of elements.
The atoms of various elements join chemically under different energy conditions to give different compounds; and all are studied under laws of chemical combination. All chemical description is well remembered when written in symbolic forms. For example, in symbolic form, Sodium + Chlorine = Sodium Chloride can be written as Na + Cl = NaCl. Similarly, Aluminium + Bromine = Aluminium Bromide can be written, in symbolic form, as Al + 3Br = AlBr3. The number of atoms joining together to give a molecule of compound formed depends upon the combining capacity of the atoms called as valency which is clearly given by a number with positive (+) or negative (–) sign shown in symbolic representation of combining substances. In the above two equations, the combining capacity of sodium and chlorine atoms is one each and therefore the equation becomes simple as Na1++ Cl1– = NaCl. But in case of second reaction, one Aluminium atom has 3 times combining capacity with Bromine and therefore one atom of Aluminium reacts with 3 Bromine atoms to give AlBr3, so the equation can be shown as 1Al+++ + 3Br– = AlBr3
Nomenclature of compounds
Learning Chemistry is easier once student gets familiar with naming of compounds, that is, the nomenclature of compounds. This topic though a big subject in itself needs brief description here, which when understood well, creates sense of satisfaction and also a sense of completeness of understanding of subject topics. There are no hard and fast rules for giving names to compounds, but in true scientific sense, the substances are named according to their constituents and their linkages. Inorganic compounds which are formed by electrovalent bonds between constituents, exist in limited numbers; and organic compounds which are formed by covalent bonds between constituents get differentiated even by change of position of same constituent in molecular space resulting in the formation of a number of compounds with same molecular formula. This property called Isomerism causes abundance of compounds in Organic Chemistry. Constituents may exist in the form of an atom or a group of atoms, dominating properties of compounds, which are called functional groups. For example,
- -F, –Cl,-Br, -I as Halides
- -CN as nitriles or cyanide
- -CHO as aldehydes
- -COOH as carboxylic acids
- -NH2 as amines, etc
Compounds are thus classified according to functional groups which each compound of the class carries as suffix to its name, e.g, inorganic compound like Metallic halides (Inorganic) – Na, K, Cl, etc., with halogen as NaCl, NaBr, KCl – Sodium Chloride, Sodium bromide; or Metallic cyanide, CN, as NaCN, KCN – Sodium Cyanide, Potassium Cyanide, etc.
+ Organic compounds like Alkyl halides or Alkyl Cyanides
Saturated & Unsaturated Hydrocarbons
Organic compounds are essentially hydrocarbons and their derivatives. Hydrocarbons are so named as being made of Hydrogen and Carbon, combined in such a way to satisfy the valencies of each combining atom. If, all carbon atoms of compound have free valencies satisfied by H-atoms, the compounds are called saturated hydrocarbons and if lesser valencies of C-atoms satisfied, the compounds are called unsaturated hydrocarbons.
The saturated hydrocarbons are named, in general, as alkanes. ‘Alk’ showing number of C-atoms and ‘ane’ showing the saturated nature of compound, for example,
1) For hydrocarbon with one C atom, 4 H-atoms will be required to satisfy the tetra-covalency of C-atom. So the formula showing one C-atom fully satisfied with Hydrogen can be CH4
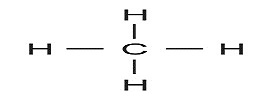
This CH4 is named as Methane, prefix ‘meth’ standing for one carbon and suffix ‘ane’ standing for saturated nature.
Similarly, for a compound with 2-C atoms, the formula will be C2H6

The two free valencies on carbon atoms joining to form bond between them.
Similarly, for each addition of C atom in chain, we can have compounds.
We can have a formula for 3-C atoms, saturated hydrocarbon as C3H8

This is named Propane where prefix is ‘Prop’ showing 3-Carbon atoms and suffix is ‘ane’ denoting the saturated nature. Thus, we can name all saturated hydrocarbons (alk-ane) changing the prefix part according to the nature of C-atoms in one molecules as Butane, Pentane, Hexane and so on.
For unsaturated hydrocarbons, with 2H atoms less, another bond is introduced between 2-atoms and the double bond thus formed shows 2H less in compound. This is represented by suffix ‘ene’ in hydrocarbon
Thus for hydrocarbon with 2H less, the formulae will be as;

This is called Eth-ene. ‘Eth’ showing number of C atoms and suffix ‘ene’ showing double bond.
If however, there is further 2H less in a compound, it will again be reflected by additional bond between the C-atoms. The triple bond is represented by suffix ‘ine’ or ‘yne’. Thus for C-atoms, we can have
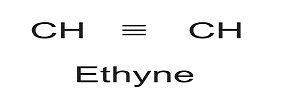
Thus from saturated hydrocarbon with 2-C atoms, we can have unsaturated form like:

Compound with more than one bond – double or triple bonds can be written as… with di, tri etc, to suffixes ad di-ene or tri-ene etc.
Cyclic compound
Similarly, the closed chain compounds are named as cyclic compound –
Cyclo-alkane
Or, cyclo-alkene
Cyclo-alkine
For example,


Double or triple bonds can be introduced into cyclo-compounds and the compounds named accordingly as cyclo-alkenes or cyclo-alkines. For example, cyclo-propane with one double bond can give a compound C3H4 and will be called as cyclo-propene. If more than one double bonds are present in cyclo-alkanes,the compounds can be named as cyclo-alk-(di,tri,tetra)-ene(s)
Similarly, cyclo-alkanes with triple bonds can be written as cyclo-alka-(di/tri/tetra)-ines
With Isomerism, the possibilities of having more number of compounds, makes nomenclature of the compounds a little more complicated and the students are advised to practice as much as possible to understand and remember well.
Do you feel strongly about something? Have a story to share? Write to us at info@thepeepertimes.com or connect with us on Facebook or Twitter